- Sulphur content
- Carbon residue
- Moisture content
2.1 Density of liquid fuel
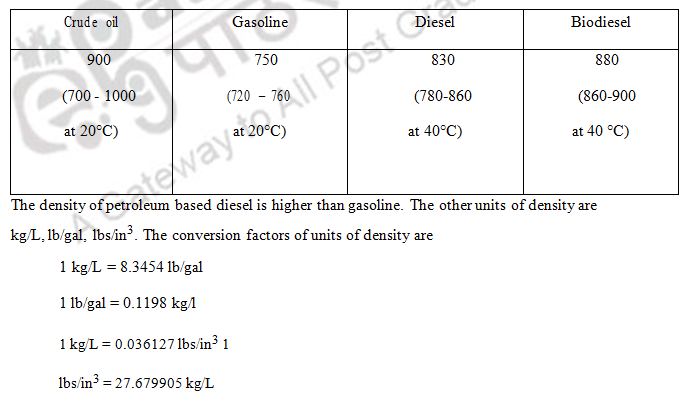
This is defined as the ratio of the mass to the volume of the fuel. It is the weight of fuel in kilogram per litre of fuel at 15°C. The density is mainly expressed in kg/m3. Hydrometer is used for measuring density of liquid fuel. It is measured at ambient temperature and converted to density at 15°C because of the expansion of liquid fuels at high temperatures and contraction at low temperatures. The difference in density from original density of a fuel indicates the contamination or adulteration of other fuel.
2.2 Specific gravity of liquid fuel
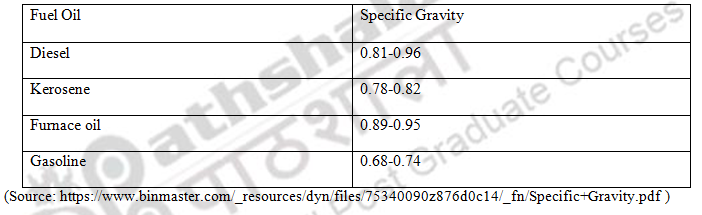
The specific gravity is the ratio of density of fuel with respect to that of water. It denotes ratio of mass to volume of fuel to that of water at a given temperature. The reference substance for liquid fuel is mainly water or liquid that is nearly water at 4°C. The pressure at which specific gravity is measured is usually 1 atm (101.325 kPa). It has no units as it is a ratio. The hydrometer is used to measure density and thereby specific gravity. Pycnometer or specific gravity bottle can also used for measurement of specific gravity. The petroleum oils are less dense than water and so specific gravity is less than one.
Table 2: Specific Gravity of Various Fuel Oils
2.3 Viscosity
Viscosity is the measure of the internal resistance of a fluid to flow. The viscosity depends on temperature and is inversely proportional. i.e. viscosity decreases as the temperature increases. So temperature has to be mentioned with viscosity values.
The fuel with high viscosity faces the problem in atomization of fuel and more chance to damage fuel injector. It also leads to incomplete combustion and poor performance of the engines. The unburned solid particles also get deposited in the engine. The fuel with low viscosity lacks sufficient lubricating power to pump and injector creating problem of leakage and much wear. So the optimum viscosity is needed for the fuel as prescribed by ASTM and EN standards.
For biodiesel, the kinematics viscosity at 40oC should be between 1.9 – 6.0 mm2/s (Standard).
The units of viscosity are Pa.s (SI unit) and centipoise (cgs).
1 Pa.s = 1000 cP & 1 cP = 0.001 Pa.s
The other units are Stokes / Centistokes, Engler, Saybolt or Redwood.
The temperature – viscosity relationship is different for different fuel. The dynamic (shear) viscosity of a fluid expresses its resistance to shearing flows, where adjacent layers move parallel to each other with different speeds (μ , mu)
The kinematic viscosity (momentum diffusivity) (ν) is the ratio of the dynamic viscosity μ to the density of the fluid ρ.
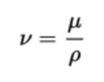
The kinematic viscosity is measured by viscometer based on the basic laws of laminar flow. The commonly used viscometers are rotating cylinder viscometer, falling sphere viscometer, capillary tube viscometer, Saybolt and Redwood viscometer. The viscosity of diesel and petrol is 2-6 cSt, 0.71 cSt respectively at 37oC and 2.71 cSt for kerosene at 20oC. The understanding of viscosity of a fuel is helpful in storage and its use.
2.4 Flash Point
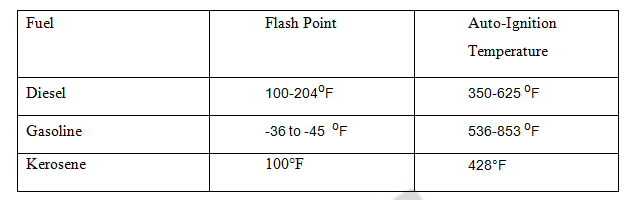
It is the lowest temperature at which the fuel evaporated to produce combustible form of gas. It refers to minimum temperature at which a volatile material can vaporize to form an ignitable mixture in air. It gives idea on safe handling of the fuel by avoiding the fire hazards. The gasoline has low flash point and is dangerous to store. The flash point is measured by flash point apparatus.
The Auto-Ignition temperature is the minimum temperature at which a fuel ignites by itself without the help of an ignition source. The Auto-Ignition temperature provides activation energy for combustion.
Table 3 Flash point and auto ignition point of diesel, gasoline and kerosene
2.5 Cloud point
The cloud point is the temperature at which the wax in the fuel forms a cloudy appearance. Above this temperature, the fuel turns to be turbid. This is important for the fuel consumption at winter. This formation of wax results in clogging of engine filters. It is also known as wax appearance point (WAP). Cloud point is measured at intervals of 2 or 3oF.
2.6 Pour point
It is the minimum temperature beyond which the fuel will not be able to pour or flow while cooling at specific conditions. It tells us about flow property under gravity and degree of pumping of fuel at low temperature. It helps to understand performance of fuel during winter. The pour point of crude oil is 278-288 K. The fuel cannot be stored at temperature below pour point. The higher paraffin content in a fuel leads to higher pour point. Pour point is measured at intervals of 5 oF. The pour point of fuels usually 10° lower than the cloud point.
Cloud and pour point apparatus is used to measure cloud point and pour point of the fuel.
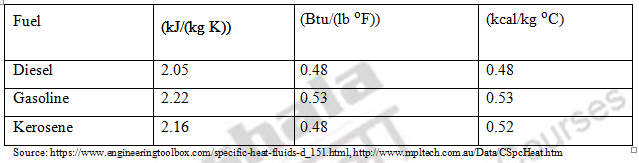
2.7 Specific Heat
It is the amount of energy required to increase the temperature of unit mass of fuel by 1o C. The unit of specific heat is kJ/(kg K) or J/(kgoC) or kcal/(kg oC) or Btu/(lbm oF). Usually the value of specific
heat of fuel varies from 0.22 to 0.28. This property tells us the amount of steam or other forms of energy required to heat fuel. The heavier the oils have higher the specific heat.
1 kJ/(kg K) = 1000 J/(kgoC) = 0.2389 kcal/(kg oC) = 0.2389 Btu/(lbm oF) Table. 4 Specific heat of common liquid fuels
2.8 Calorific Value
The calorific value is the quantity of energy generated from the complete combustion of fuel. It is measured either as gross calorific value or net calorific value.
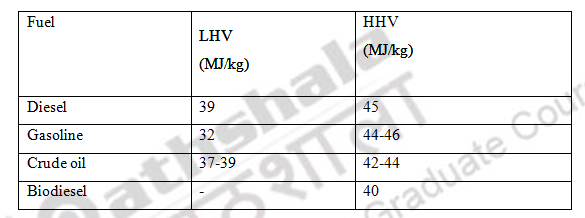
- Gross calorific value (GCV) refers to energy produced during the combustion process including steam or water vapour. It is also known as high heating value (HHV) or gross energy.
- Net calorific value (NCV) refers to the energy produced by the combustion deducting the water vapor. It is also known as low heating value (LHV)
It can be explained with the following example. A hydrocarbon fuel with C, H, S the combustion products are CO2, H2O and SO2. The reference state of combustion products
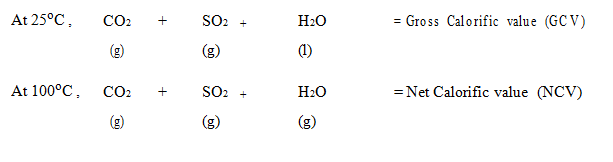
The units of calorific value are
cal/g or kcal/kg or kJ/kg or Btu/lb or cal/gmol or kcal/kgmol or kJ/kgmol or Btu/lbmol
So Gross Calorific value is always greater than Net Calorific value by an amount equal to latent heat of condensation. Fuel efficiency is mainly depends on the net calorific value. The calorific value is measured by bomb calorimeter.
Table 5 Calorific value of common fuels
2.9 Vapour pressure ( pv ) : It is defined as the pressure exerted by vapour in phase equilibrium with its liquid at a given temperature. For a pure substance, it is same as the saturation pressure.
Table 6 Vapour pressure of common fuels

2.10 Sulphur content
It is the amount of sulphur present in the fuel. The sulphur content is mainly based on source of crude oil and process of refining. The sulphur content in a fuel leads to the risk of corrosion with the
formation of sulphuric acid after condensation. The Sulphur content of kerosene is 0.05-0.2 percent while the furnace oil has higher Sulphur content of 2-4 percent.
2.11 Ash Content
It refers to the inorganic material present in the fuel. The residual fuels produced after distillation of gasoline and diesel has high ash-forming constituents like salts of sodium, vanadium, calcium, magnesium, silicon, iron, aluminum, nickel, etc. The high ash content in liquid fuels results in deposition of it in combustion equipment. It also has the erosive effect on the burner tips at high temperature. The ash value of the fuel varies from 0.03-0.07 percent. The ash content is important in the design of the furnace grate, combustion volume, pollution control equipment and ash handling systems of a furnace.
2.12 Carbon Residue
It is the amount of carbonaceous solid residue present in the fuel. The deposition of solid carbonaceous residue after evaporation of vaporizable constituents is due to this property of the fuel. Normally residual oil contains carbon residue of 1 percent or more.
2.13 Moisture Content
A maximum limit of 1% moisture is specified in the standards of liquid fuel. It refers to the water content in the fuel. The water and dissolved salts in it damages the inside furnace surfaces during combustion. The flame spluttering at the burner tip, flame ceased burning and reduction of flame temperature or lengthening of flame are some of the problems due to water conten tin fuel either rin free or emulsified form.
2.14 Chemical Composition of Crude oil
The crude oil consists mainly of saturated hydrocarbons, aromatics, resins.
Table 7 Chemical composition of crude oil

2.15 Octane number is an index for estimating the resistance of fuel to autoignition. It relates to anti-knocking property of a fuel. This is a measure in a spark-ignition engine. It is defined by comparing the fuel at test with the mixture of iso-octane and normal heptane that have the same anti-knocking capacity. For example, gasoline with the same knocking characteristics as a mixture of 95% iso-octane and 5% heptane would have an octane rating of 95. Gasoline has octane number ranged from 92-98.
2.16 Cetane number is a measure of a fuel’s ignition quality in compression-ignition engines. It denotes delay between fuel injection into the combustion chamber and the beginning of compression ignition (auto-ignition). So it is a more important for ignition quality of diesel fuel. The higher the value of cetane number of a fuel, easier will be the starting of the engine. It is defined by comparing fuel with that of a mixture of cetane and methylnaphthalene that have the same ignition quality. The fuel with larger cetane numbers has lower ignition delays. The Cetane number is only valid for light distillate fuels. Cetane number of diesel is 45 (between 40-55) and that of biodiesel is 60-65. Cetane number is irrelevant in typical gasoline applications (spark ignition).
The most important properties of a solid fuel are as follows
- Calorific value
- Sulphur content
- Moisture content
The major solid fuels are coal and wood.
3.1 Density of solid fuel
The density of wood or coal is also the mass of fuel per unit volume of fuel. The density of wood depends on moisture content and porosity. Maximum is for ρcellulose – 1550 and very soft wood has 50 kg/m3.
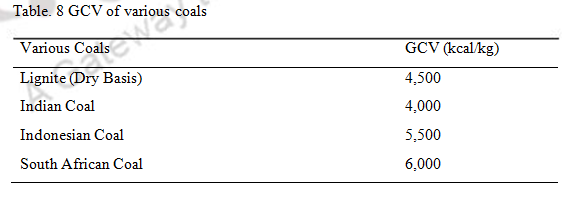
3.2 Calorific value/ Heating Value: The heating value of coal is an important thermal property. The Gross Calorific Value (GCV) are different for coal of different origin.
Table. 8 GCV of various coals
The Indian coal is graded into seven grades namely A,B, C,D,E and F based on calorific value.
Table. 9 Calorific value of Indian coals
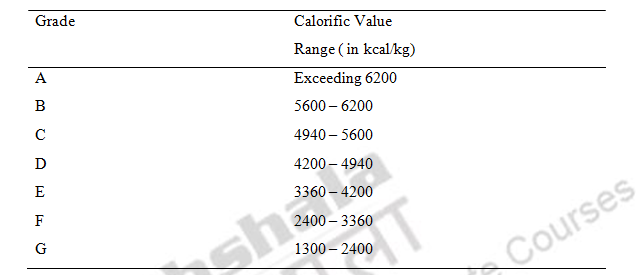
Normally D,E and F coal grades are available to Indian Industry. The chemical composition of coal has a strong influence on its combustibility. The calorific value of coal varies considerably depending on the ash, moisture content and the type of coal while calorific value of fuel oils is much more consistent.
The calorific value/HHV of coal and wood are as follows:
Table. 10 HHV of coal and wood
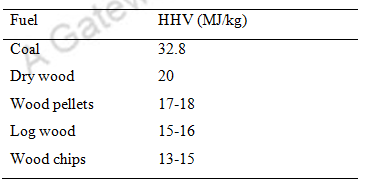
The chemical composition of coal is as follows:
Table. 11 Chemical composition of Coal
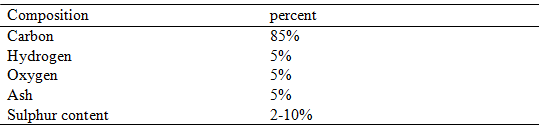
3.3 Fixed carbon is the carbon content in its free state. This is the solid combustible residue after expulsion of volatile matter in a combustion process. The fixed carbon is measured by thermogravimetric analysis (TGA). The heating value of the fuel is mainly depend on fixed carbon content.
3.4 Volatile matter refers to those combustible constituents of coal that vaporize when coal is heated. It is measured from weight loss after heating fuel at higher temperature of 900 oC for 15 minutes in a muffle furnace. Higher the volatile matter, the fuel will be ignited easily.
3.5 Moisture content
The anthracite coal and lignite have moisture content of 5-15% and 30-60% respectively. The moisture content in wood ranges 20-80%. The moisture content is measured from weight loss of sample at a temperature of 105 oC in hot air oven.
There are two methods of analysis of coal.
- Proximate analysis determines only the fixed carbon, volatile matter, moisture and ash percentages.
- Ultimate analysis is the quantitative estimation of different elements present in a fuel sample Ultimate analysis: water + percentage of C, H, O, N, and S
This ultimate analysis provides most comprehensive results. The ultimate analysis is determined in a properly equipped laboratory by a skilled chemist, while proximate analysis can be determined with a simple apparatus.
4.1 Proximate Analysis
Proximate analysis indicates the percentage by weight of the Fixed Carbon, Volatiles, Ash, and Moisture Content in coal. The amounts of fixed carbon and volatile combustible matter directly contribute to the heating value of coal.
From the proximate analysis of coals of Indian, Indonesian and South African origin, it is clear that fixed carbon content of Indian coal is 34.69 percent which is comparatively lesser than South African coal (51.22 percent) and Indonesian (46.79 percent) coal. The ash content in Indian coal is 38 percent and in Indonesian and African coal are 13 and 17 percent respectively. The moisture content and volatile matter in Indian coal is 5.98 and 20.7 percent was also lower than Indonesian and South African coal.
4.2 Ultimate Analysis
Elements like Carbon, Hydrogen, Oxygen, Sulphur, etc. are estimated in ultimate analysis of a fuel sample. This is helpful to determine amount of oxygen required for combustion and the volume and composition of the combustion gases. Ultimate analysis of Indian coal reported carbon, hydrogen, nitrogen, sulphur and oxygen content of 41.11 percent, 2.76 percent, 1.22 percent, 0.41 percent and 9.89 percent respectively.
4.3 How Ultimate Analysis and Proximate Analysis are related?
The following relationship is applicable for coal with more than 15% moisture.
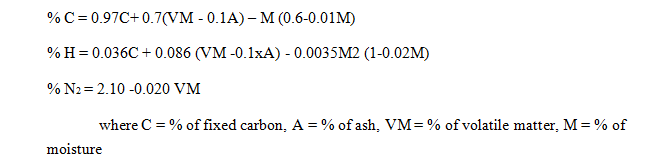
- Properties of Gaseous Fuels
The properties of gaseous fuel are
- – Specific gravity: Lighter than Air. For example Natural gas: 0.6-0.8.
- – Autoignition temperature of natural gas: 537-540oC
- – Dynamic viscosity: Natural gas behaves like a Newtonian fluid
- – Flammable: Natural gas has a flame temperature of 1954 o
- – The most important property of a gaseous fuel is the calorific value. Compared to solid and gaseous fuel is more efficient and has high calorific value.
The amount of energy present in a gaseous fuel is the calorific value of gaseous fuel. It is normally expressed as kilocalories per normal cubic meter (kCal/Nm3). The normal indicates normal temperature (20o C) and pressure (760 mm Hg). The high heating value of natural gas is 9350 kcal/Nm3.
- Compositional analysis of a gas mixture by Orsat apparatus
The composition of fuel gas and byproducts from fuel combustion is measured with the help of Orsat apparatus. The gas sample is passed to the pipettes one by one, by opening the stopcock and raising and lowering the aspirator bottle several times for ensure proper adsorption of gases in the respective adsorbents. The decrease in volume from the initial volume after passage through every pipette gives the account of volume percent of the respective gas. This apparatus is mainly used to estimate composition of flue gas.
Conclusion
The physico chemical properties of the fuel are very significant for its use in engines and energy production. An ideal fuel is the one with high calorific value, moderate ignition temperature, low moisture content and less emissions of SOx and NOx and other toxic gases.
Energy and environment Copyright © by Dr. Dhanya M.S M.S. All Rights Reserved.